Chemistry Words: Ionic bonding
Simple Description
A type of bond formed between positive and negative ions
Further Detail
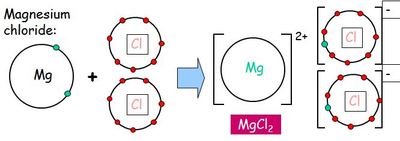
For example, in sodium chloride an electron is "given" from the outermost electron shell in sodium to the outermost electron shell in chlorine. This turns the sodium atom into a sodium ion (positive charge) and the chlorine atom into a chloride ion (negative charge). The two ions are attracted to each other due to their opposite charges - an ionic bond. Ionically-bonded substances are usually solids at room temperature due to the strong electrostatic forces between the ions, lots of heat energy will need to be added to overcome these forces and melt it.
Related Words:
« Previous Word Next Word »
