Chemistry Words: Titration
Simple Description
Titration is a method used to determine the concentration of an unknown acid or alkali
Further Detail
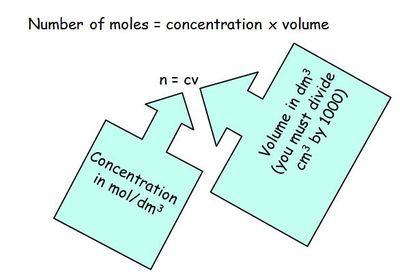
Titrations are carried out using a pipette to measure a certain volume of alkali into a conical flask. An indicator is added so that the end point can be identified. If an alkali has been added to the conical flask an acid is added to the burette. The acid is added slowly until the last drop to cause a colour change is added. The volume of acid added is recorded and repeat measurements are taken until you have readings which are within 0.1 cm3 of each other. The equation n=cv (n= number of moles, c= concentration in mol/dm3 and v= volume in dm3, note you will record the volume of acid from the burette in cm3 so figure must be divided by 1000 to convert to dm3) is used to calculate the number of moles and then the moles and volume is used to calculate the concentration of the unknown alkali.
Related Words:
« Previous Word Next Word »
